The first of the three classical laws of thought is lex identitatis or the law of identity. In logic, the law of identity states that each thing is identical with itself. By this it is meant that each thing (be it a universal or a particular) is composed of its own unique set of characteristic qualities or features, which the ancient Greeks called its essence. (Wikipedia)
In the literature on chemistry two principles, two ways of electronic formulae recording are adopted:
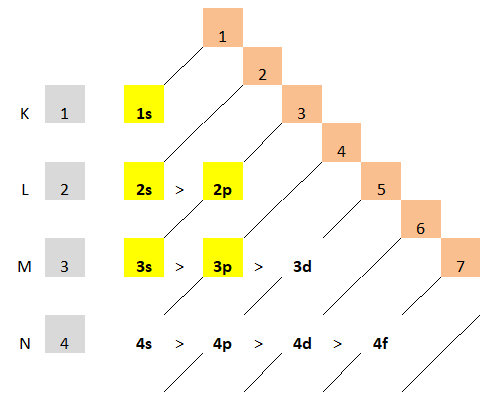
The first principle reflects the placement of electronic levels and sublevels of the hydrogen atom in Niels Bohr’s model:
K L M N O
1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, 5f, 5g,
The second principle of minimum energy determines the order of filling of atomic orbitals having different energies. According to the principle of minimum energy, electrons are primarily occupied by orbitals having the lowest energy. The energy of the sublevels grows in a series:
1s <2s <2p <3s <3p <4s <3d <4p <5s <4d <5p <6s <4f <5d <6p <7s <5 f <6d …
The rule of Klechkovsky (1951) – Madelung (1936) – Janet (1930):
The electrons fill the orbitals in the atom in the order of increasing sum of the principal and orbital quantum numbers n + l. For the same amount, an orbital with a smaller value of n is filled earlier.
Both principles are clearly represented in the generally accepted triangular table:
Bohr’s principle reads the table along horizontal lines from left to right. The Klechkovsky-Madelung-Janet principle reads the table from the right to the left obliquely from the top down.
In other words, for a given configuration, the order of writing the orbitals is not strictly fixed since only the orbital occupancies have physical significance. For example, the electron configuration of the titanium ground state can be written as either [Ar] 3d2 4s2 or [Ar] 4s2 3d2. The first notation groups all orbitals with the same value of n together, corresponding to the “spectroscopic” order of orbital energies that is the reverse of the order in which electrons are removed from a given atom to form positive ions; 3d is filled before 4s in the sequence Ti4+, Ti3+, Ti2+, Ti+, Ti. The second notation follows the order based on the Klechkovsky-Madelung-Janet rule for the configurations of neutral atoms; 4s is filled before 3d in the sequence Ar, K, Ca, Sc, Ti.
Attention! The modern table of the Periodic Law begins according to the first principle, that is, according to the model of the Bohr’s atom of hydrogen. This is indicated by the numbering of the periods and the placement of the modern 1st and 2nd groups of s-elements on the left edge of the “rectangular” table. Contradictions to this scheme do not arise up to 3p-elements, i.e. up to an element with the charge of the nucleus 18 inclusive. Then there is a barely noticeable absence of 3d after 3p, then a noticeable appearance of 3d after 4s, and so on. These signs indicate that in the process of going through the table we unconsciously switched (!) from reading the table from left to right horizontally to reading it from the right to the left going oblique from top to bottom, switching from the Bohr’s principle to the aufbau principle of minimum energies.
De facto, the second way of reading, like the first, is fixed in the numbering of the modern table periods, whose lengths are pairwise equal. There is a violation of lex identitatis – the first law of logic.
In addition to subjective factors of non-scientific nature, the error could be facilitated by the perfect coincidence between the first five terms of both sequences.
1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 5f, …
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d …
The violation of the law of identity in a particular case faces no obvious consequences due to the equality l = 0 for s-elements. With an arbitrary displacement of the s-block in the table, the sum (n + l) for it does not change.
This note about violation of the logical law is in no way to diminish or to detract from the greatness of the Periodic Law of D.I. Mendeleyev and Quantum Theory. It’s just about putting things in the ledger in order.
The error was corrected (without direct indication of this error) by the French scientist Charles Janet in 1929. In the table he had proposed, the sum (n + l), i.e. the first step in the Klechkovsky rule, was stitched in the numbering of periods. Janet’s table was repeatedly rediscovered by various scientists, which confirms the rationality of the approach, but had never been accepted by the scientific authorities.
The re-enumeration of periods by Janet does not affect the number of elements in the table or the quantum numbers of electrons in atoms. And it does not affect the fundamental interpretation of the periodicity of chemical properties from the standpoint of chemistry and quantum mechanics. So, to use a well-known analogy, the renumbering of floors (periods) in a apartment house (table) does not affect the numbering of apartments (subshells), the arrangement of rooms (elements) and the behavior of tenants (electrons) in these apartments.
On the other hand, the choice of the form for the periodic system, as for any scientific abstraction, is far from being just a matter of taste. Irregularity in the pairing of the lengths of periods causes a cognitive dissonance and encourages finding a pair for the first period. Thus, Dmitry Ivanovich Mendeleyev himself suggested the existence of two elements of the zero period: Newtonium and Coronium. However, he repeatedly stressed their hypotheticalness and did not include them in the tables of the elements of the 7th and 8th editions of the Fundamentals of Chemistry. Note that the manifestation of a double (triple, quarter, etc.) standard in the recording of periodic phenomena is not very rare and may not lead to fatal consequences. So, fractions of a second are measured in the decimal system, seconds and minutes – in the sexagesimal, hours – in the twelve or twenty-four-hour system (day), day – in the septenary (weeks) and about-thirty (months), etc. number system. In calendar systems, this is dictated, at least, by the cycles of the moon and the sun, historically formed by number systems. In addition, on timelines, the presence of a double standard is conscious. It is always possible to go from one number system to another. In the modern periodical system, the presence of a double approach is masked, depriving it of mathematical logic.
What precludes us from adopting a single standard in the record of the periodic law of the elements of Dmitry Ivanovich Mendeleyev?
Literature:
- http://www.ipgp.fr/~tarantola/Files/Professional/Mendeleev/Janet_1929.pdf
- http://secology.narod.ru/mon_and_di.html